Tag: generic drugs

Training Pharmacy Technicians: Mastering Generic Drug Competency Standards
Finnegan O'Sullivan Feb 23 7Pharmacy technicians must master generic drug knowledge to prevent life-threatening errors. With 90% of prescriptions being generics, competency in drug names, appearances, and classifications is non-negotiable for patient safety and certification.
More Detail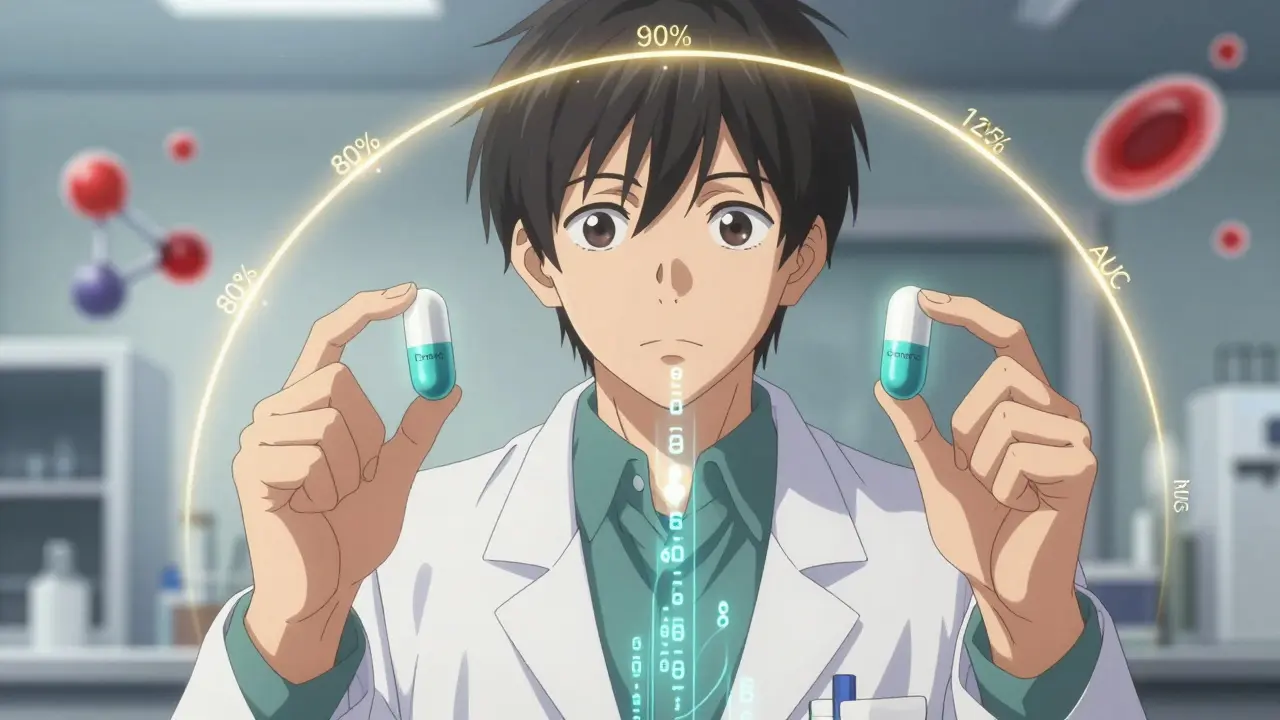
Bioequivalence Studies: What the FDA Requires Generic Drug Manufacturers to Prove
Finnegan O'Sullivan Jan 23 14The FDA requires generic drug manufacturers to prove bioequivalence through rigorous clinical studies that show their product delivers the same active ingredient at the same rate as the brand-name version. This ensures safety, effectiveness, and cost savings for patients.
More Detail
The 80-125% Rule: Understanding Bioequivalence Confidence Intervals in Generic Drugs
Finnegan O'Sullivan Jan 10 8The 80-125% rule ensures generic drugs work like brand-name versions by comparing how much drug enters the bloodstream. It's not about pill strength - it's about absorption. Here's how it works and why it matters.
More Detail
Out-of-Pocket Costs: How Generics Slash Your Medication Bills
Finnegan O'Sullivan Jan 3 14Generic drugs cut prescription costs by up to 90%, yet most people still overpay due to opaque pricing. Learn how to save hundreds annually by switching to generics and using direct-to-consumer pharmacies.
More Detail
Healthcare System Savings: How Generic Drugs Cut U.S. Drug Costs by Hundreds of Billions
Finnegan O'Sullivan Jan 1 15Generic drugs saved $482 billion in U.S. healthcare spending in 2024, making up 90% of prescriptions but just 12% of costs. Biosimilars are the next frontier in cutting drug prices - if we remove the barriers holding them back.
More Detail